Abstract
Background.
Studies have suggested that chronic autoimmune diseases and infections increase the risk of myeloid malignancies. However, due to the strong association between exposure to cytotoxic agents and subsequent acute myeloid leukemia (AML) and a lack of clinical data in previous studies, it remains unclear whether the reported risk is caused by e.g. cytotoxic and immune modulating agents given to treat a variety of autoimmune diseases.
Methods
To investigate if acute and chronic inflammation per se increase the risk of AML or whether this association are explained by preceding cytotoxic therapy or hematological diseases, we conducted a nationwide case-control study using the Danish National Acute Leukemia Registry. We identified 3165 AML patients diagnosed 2000-2013 Patients were matched to population controls on sex and age through the Danish National Registry of Patients. To avoid recall bias, we retrieved individual-level information on inflammation measured as autoimmune disease, infections, and use of antibiotics through the Danish National Registry of Patients and the Danish National Health Service Prescription Database.
We used conditional logistic regression analysis to compute odds ratios for AML. To avoid reverse causation, we ignored exposures ≤3 years before AML diagnosis/index date. Results were stratified by AML type (de novo AML, secondary AML or therapy-related AML), sex, and age. Results were given with 95% confidence intervals (CI).
Results
The median age was 68.8 years (range 15-99) and 55% of the cases were men. Among cases, 74.1% had de novo AML, 19.1% had sAML, and 6.8% had tAML. Only 3.5% had APL.
Overall, 239 (7.6%) of patients and 1847 (5.8%) of controls had an autoimmune disease diagnosis more than 3 years before their AML diagnosis or the index date. Having an autoimmune disease was associated with an overall increased risk of AML (OR 1.3 (CI=1.2-1.5)). However, the risk was only increased for sAML and tAML (OR 2.1 (CI=1.7-2.6)), and not for de novo AML (OR 1.1 (CI=0.9-1.3)).
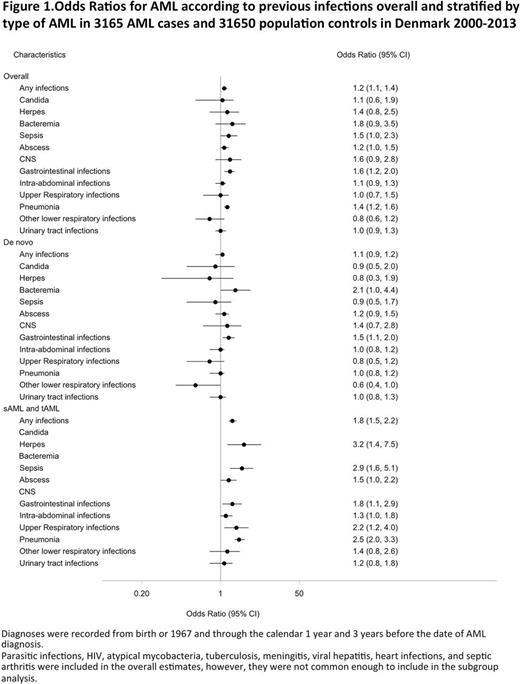
The association between infections and AML overall and by AML type is shown in Figure 1.
Any hospitalization-requiring infection was associated with a higher risk of AML (OR 1.2 (CI=1.1-1.4)). Stratified by type of AML, this association was explained by a high risk of sAML/tAML (OR 1.8 (CI=1.5-2.2)) and not by de novo AML (OR 1.1 (CI=0.9-1.2)).
To capture community-acquired infections, we used antibiotic prescriptions. Overall, 2539 (80.2%) of patients and 24,676 of controls had redeemed more than one prescription for antibiotics >3 years before their AML diagnosis/index date. Prior use of antibiotics was associated with a higher risk of AML (OR 1.2 (CI=1.1-1.3)). However as for infections, the association was only evident for sAML/tAML (OR 1.8 (CI=1.5-2.2)), whereas the risk of de novo AMLwas not increased (OR 1.0 (CI=0.9-1.1)).
In a subgroup analysis of individual autoimmune diseases, only gastrointestinal disease was associated with de novo AML (GI autoimmune disease: de novo AML: OR 1.5 (CI=1.0-2.1), sAML/tAML: OR 1.5 (CI=0.9-2.7)). Of infections, only gastrointestinal infections were associated with de novo AML (de novo AML: OR 1.5 (CI=1.1-2.0), sAML/tAML: OR 1.8 (CI=1.1-2.9)).
Conclusion
This large population-based study using high-quality data shows that use of antibiotics, infections, and autoimmune diseases are not per se associated with AML.
Only gastrointestinal autoimmune disease and gastrointestinal infections were associated with increased risk of de novo AML. Though, we cannot rule out the possibility that infections or autoimmune diseases in the gastrointestinal tracts cause immune-related/inflammation-driven leukemogenesis, these findings are likely coincidental or explained by therapeutic immune-modulating agents increasing both risk of infections and AML.
In summery, our results indicate that in patients without prior hematological disease or exposure to cytotoxic therapy, inflammation plays - if any - only a minor role in leukemogenesis.
Østgård: Pfizer: Other: Travel expences; The Danish Cancer Society: Research Funding. Nørgaard: Program for Clinical Research Infrastructure (PROCRIN): Research Funding. Pedersen: Program for Clinical Research Infrastructure (PROCRIN): Research Funding. Østgård: Lilly: Consultancy; Bristol-Myers Squibb: Consultancy; Pfizer: Consultancy; AbbVie: Consultancy. Schöllkopf: Pfizer: Other: Travel expences; Teva: Other: Travel expences; Karyopharm Therapeutics: Research Funding. Severinsen: ARIAD: Research Funding; Celgene: Research Funding; Novartis: Research Funding. Jensen: The Dagmar Marshall Foundation: Research Funding; The Danish Acute Leukemia Group: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal